Sugar Solubility
Let’s kick things off with a graph.
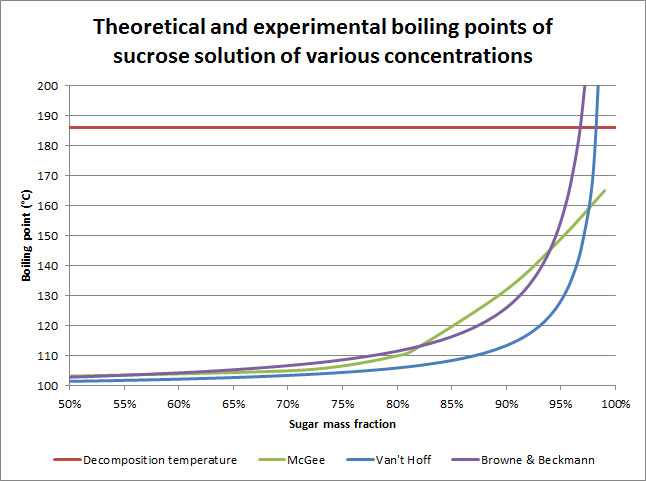
Just a note: when calculating sugar concentration, you divide the amount of sugar by the amount of solution, not of water. So the maximum concentration is just under 100% (since there would be no water at 100%), even though you can dissolve 2 parts sugar into 1 part water at room temperature.
This graph shows the boiling point of a sugar solution based on its concentration. Notice that each boiling temperature is associated to a single concentration. That’s why we use thermometers. That’s also why we can add water and start over if ever we screw up.
Another thing worth noting is that the temperature increases exponentially faster past 85% or so. These are also the cool concentrations because they translate to candy instead of syrup. The increase in concentration is fairly linear because the water will evaporate at a pretty constant rate. What this means for us in the kitchen is that we’ll need to be especially careful once our syrup reaches those higher concentrations because it will be easier to burn it.
McGee’s curve is experimental- the other two are theoretical. So the increase in temperature isn’t quite as dramatic as theory would have us expect, and we’ll get into why later.
Incidentally, I got the Browne curve from a chemistry book published in 1912 called The Handbook of Sugar Analysis. One chapter deals with artificial sweeteners and mentions the use of lead acetate. They stopped using it when people started dying of lead poisoning.
The solubility of sugar is one also of the factors that make it so well suited to candy-making. Consider a chewy piece of caramel. It’s chewy because there’s still water in it, even though it’s not a puddle. You can sort of compare it to a sorbet (because I like sorbet analogies): in sorbet, the sugar “binds” some of the water by forming a highly concentrated solution that cannot freeze. So you effectively have liquid that’s dispersed between many (ideally) tiny ice crystals. Caramel is the other way around- you have water that’s keeping the sugar crystals from forming a solid mass.
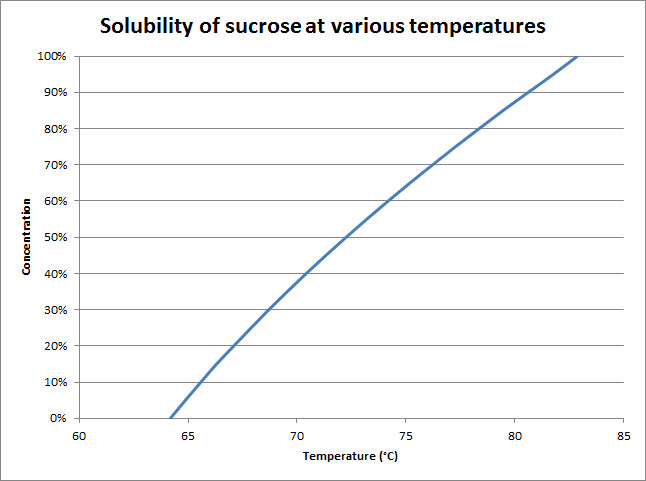
What happens in fact is that the solubility of sugar reaches a sort of equilibrium. A sugar molecule will move between two states, depending on the movement of water molecules in the candy: a ‘hydrated’ or dissolved phase, and a ‘crystallized’ or solid phase.
When the caramel base is really hot, it’s quite runny because a lot of it is dissolved since its solubility is very high. As the syrup cools, the solubility drops and the sugar is forced out of the solution. This is where undesirable crystallization can occur, for example, when making fudge. What we’re left with is solid sugar with small amounts of saturated sugar solution in it. How much water is left in the candy can be used to determine water activity, which is completely useless for the home cook, but many chefs could use to determine how shelf-stable their candy is. It’s especially important with candy that also has fat.
You’ll always have a liquid part and a solid part. Like a lot of foods really. Candy is so simple in terms of ingredients that it’s great for understanding things like ganache or sorbet.
To illustrate this, let’s make pralines! I fell in love with these on a recent trip to New Orleans. Pralines are in crystalline solid – like fudge, or salt, or quartz. Cooking slowly increases the amount of sugar inverted, which makes the pralines set slower and softer.
- Pralines
- 3 cups pecans, chopped
2 cups dark brown sugar
1 cup sugar
1 cup cream
1 cup milk
½ cup or 1 stick unsalted butter
½ tsp salt
1 tsp vanilla extract
Combine all ingredients in a pot at least twice the volume of all the ingredients and cook to the softball stage (225°F – 230°F) over medium low heat. Remove from the heat and cool 7 minutes. Stir until the pralines take on a slightly opaque appearance and begin to crystallize on the edge of the pan. Quickly spoon on to parchment paper.
Just for the fun of it, set aside a spoonful of the solution before mixing it and compare it to the cooled praline. That’s crystallization.